- Treatment Preference Matrix
- Dr Love's Email
- Video Clip from MM Working Group*
- Video Presentations
- CME Test
- PowerPoint slides
- CME information and faculty information
- Nikhil C Munshi, MD
- Sagar Lonial, MD
- Guido Tricot, MD, PhD


Like most other current corners of medical oncology, multiple myeloma has been the subject of a litany of published consensus papers and clinical guidelines. However, the recent (and continued) explosion of related research findings has resulted in multiple “acceptable” evidence-based options and has sometimes rendered these sources confusing and of suboptimal assistance in making “real-life” decisions.
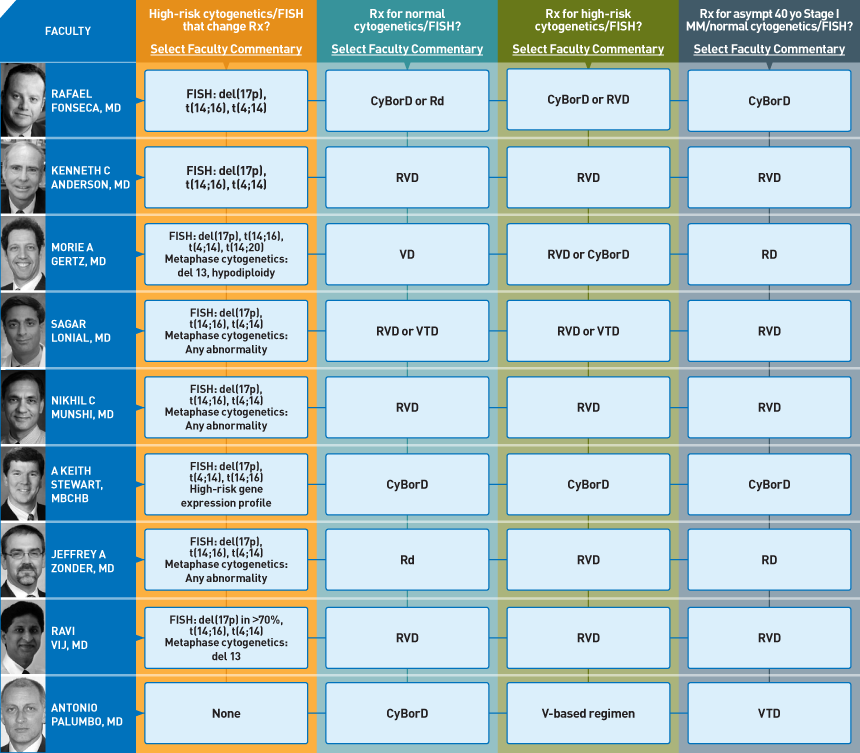
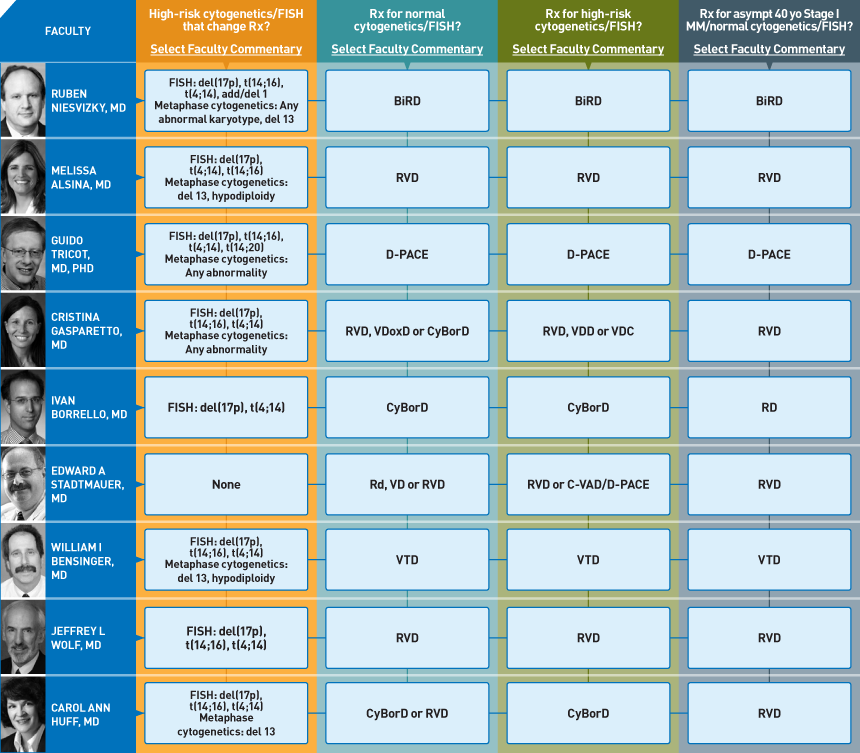
To help address this important unmet need, on April 7 and 8 our CME group brought together 19 multiple myeloma clinical investigators and 19 oncologists to delve into an underdiscussed and underpublished topic — the usual management choices made in clinical practice. Our goal for this initiative was to dissect dozens of important clinical decisions and say to the participants, “We all know there are multiple rationale options, but reflecting back on your own practice, what do you USUALLY do?” To efficiently summarize the conclusions, we developed a simple-to-read graphic — the Treatment Preference Matrix (TPM — click here for this issue).
To execute this ambitious undertaking, the extraordinary day-and-a-half “Multiple Myeloma Working Group” had two major elements woven into the unforgettable discussion: 1) 10-minute slide reviews by each investigator of specific treatment issues (click here for three video presentations related to this week’s topics) and 2) Presentation of cases (24) from the practices of the community oncologists that is currently being edited into an internationally distributed audio program.
Throughout the meeting we spent hours trying to get at the heart of these complex decisions and each participant not only shared their thoughts verbally, but also provided their perspectives and experiences using a laptop computer networked to our oncology chat room and comment collection database. The entire event was audio and video recorded, and everything we gathered will be used in the development of an array of educational activities in multiple formats.
This, the first of eight “highlight film” emails, marks the launch of our integrated curriculum and focuses on three common and critical questions in clinical decision-making:
What cytogenetic and FISH findings are important enough to change treatment algorithms?
This question resulted in the most ambiguous conversation during the meeting, and a number of the practicing oncologists stated they came away even more confused after the extended discussion. Although there was general agreement on a number of specific indicators of adverse prognosis, including t(4;14), t(14;16) and del(17p) by FISH and del13 and hypodiploidy by cytogenetics, many other areas of major disagreement emerged, and ongoing research is vigorously attempting to further clarify some of these muddied waters.
What is your usual induction regimen in a younger transplant-eligible patient with normal FISH and cytogenetics?
Among the 19 investigators, at least six different regimens are “preferred.” However, most are opting for “triple treatment” including dexamethasone, bortezomib and an IMiD® — usually lenalidomide. Along these lines, the RVD regimen (lenalidomide, bortezomib and dexamethasone) is perhaps most frequently used but another common choice with a similar theme is CyBorD (cyclophosphamide, bortezomib, dexamethasone), and some of these investigators integrate an IMiD on the back end in the form of post-transplant lenalidomide maintenance (see next issue).
What is your usual induction regimen in a younger transplant-eligible patient with high-risk FISH and/or cytogenetics?
Although there is controversy regarding what constitutes high risk, there is agreement that a proteasome inhibitor — specifically bortezomib — should be part of the induction mix. Of note, because many investigators are already using that agent as part of a doublet or triplet anyway (see #2) risk status ends up having less of a major impact on the selection of induction, although it remains an important consideration in the timing and type of transplant- and post-transplant maintenance.
A case in point (presented by Dr Lyle Feinstein):
Cases of young patients are always provocative, and in this case, a 40-year-old woman presented with ISS Stage I disease and anemia but no other cytopenias or bone disease. The disease was considered (after much discussion) to be normal-risk. Click here for a two-minute video clip of some high points of the conversation surrounding this case, and click here for the TPM of the entire group.
Next up on this series: More perspectives and controversy as we uncover the faculty’s preferences for timing and method of transplant and post-transplant maintenance treatment.
Neil Love, MD
Research To Practice
Miami, FL
How would you manage a 40-year-old patient with asymptomatic ISS Stage I multiple myeloma and normal cytogenetics/FISH?
 |
Rafael Fonseca, MD |
| Consultant, Professor of Medicine
Mayo Clinic Arizona
Deputy Director, Mayo Clinic Cancer Center
Scottsdale, Arizona |
 |
Edward A Stadtmauer, MD |
| Professor of Medicine
Leader, Hematologic Malignancies Program
Director, Bone Marrow and Stem Cell Transplant Program
Division of Hematology-Oncology
Abramson Cancer Center of the University of Pennsylvania
Philadelphia, Pennsylvania |
I would use RVD for four cycles and then enroll the patient on the BMT CTN 0702 trial, which is evaluating a single autotransplant with lenalidomide maintenance versus two autotransplants followed by lenalidomide maintenance versus a single autotransplant followed by RVD for four cycles followed by lenalidomide maintenance.
I believe the BMT CTN trial will provide insight on this type of patient. Based on the data that we have, this patient does not benefit from the tandem autotransplant followed by nonmyeloablative allotransplant. I would HLA-type the patient and see how well she responds to therapy and use those responses to determine whether or not I might consider her for an allotransplant.
 |
Ruben Niesvizky, MD |
| Associate Professor of Medicine
Director, Multiple Myeloma Service
Department of Medicine
Division of Hematology/Oncology
Weill Cornell Medical College
NewYork-Presbyterian Hospital
New York, New York |
 |
Jeffrey A Zonder, MD |
| Associate Professor of Medicine and Oncology
Karmanos Cancer Institute
Wayne State University
Detroit, Michigan |
 |
William I Bensinger, MD |
|
Professor of Medicine, University of Washington |